Now it's derm devices' time to shine
Also: Organon writes a cheque with 9 zeros for admission to the dermasphere; Musely expands in teledermatology; the coming boom in tattoo-removal procedures; and much more
Welcome to the dregs-of-September edition of Dermatology.Business, where we savour every last minute of what someone in an undergraduate Eng Lit class once called “this season of fruity mellowness.” You’ll find us here in this same location every other Wednesday, harvesting every development of interest from the Dermasphere. Be a pal and share this bounty of harvested news with your teams and colleagues, why don’t you?
According to a new report, the global market for dermatology devices is projected to nearly double by 2030.
By the numbers
Recent market size (2023): Cdn$18.2 billion (US$13.5 billion)
Projected market size (2030): Cdn$34.6 billion (US$25.7 billion)
Expected CAGR: 9.7%
Photo by Safia Shakil
Driving the trend
Aging population seeking cosmetic and anti-aging treatments
Increased awareness of skin health due to environmental factors
Rising disposable income and spending on wellness and aesthetics
Technological advancements in device manufacturing
Growing prevalence of skin disorders
Key players: 3Gen, Inc., Bruker Corporation, Carl Zeiss A/S, among others
What to watch: The U.S. market was estimated at Cdn$4.85 billion (US$3.6 billion) in 2023, while China is forecasted to grow at 13.7% CAGR, reaching Cdn$7.7 billion (US$5.7 billion) by 2030.
The bottom line: With factors such as A.I. integration, telemedicine growth, and increasing healthcare expenditure in emerging markets, the dermatology devices sector is poised for significant expansion.
UCB scores triple FDA approval for BIMZELX
The FDA approved UCB's bimekizumab-bkzx (BIMZELX) for three new indications: active psoriatic arthritis, active non-radiographic axial spondyloarthritis, and active ankylosing spondylitis.
Why it matters: BIMZELX is now the first and only IL-17A and IL-17F inhibitor approved in the U.S. for four chronic immune-mediated inflammatory diseases, including its previous approval for moderate-to-severe plaque psoriasis.
By the numbers
In psoriatic arthritis trials, 43-44% of patients achieved ACR50 response at Week 16 vs. 7-10% for placebo
In axial spondyloarthritis trials, 45-48% achieved ASAS40 response at Week 16 vs. 21-23% for placebo
The big picture: These approvals expand treatment options for patients with these chronic inflammatory conditions and strengthen UCB’s position in the competitive immunology market.
Whats next: UCB will focus on the stateside commercial launch and market access for these new indications. The company aims to make BIMZELX available to eligible patients while navigating affordability challenges.
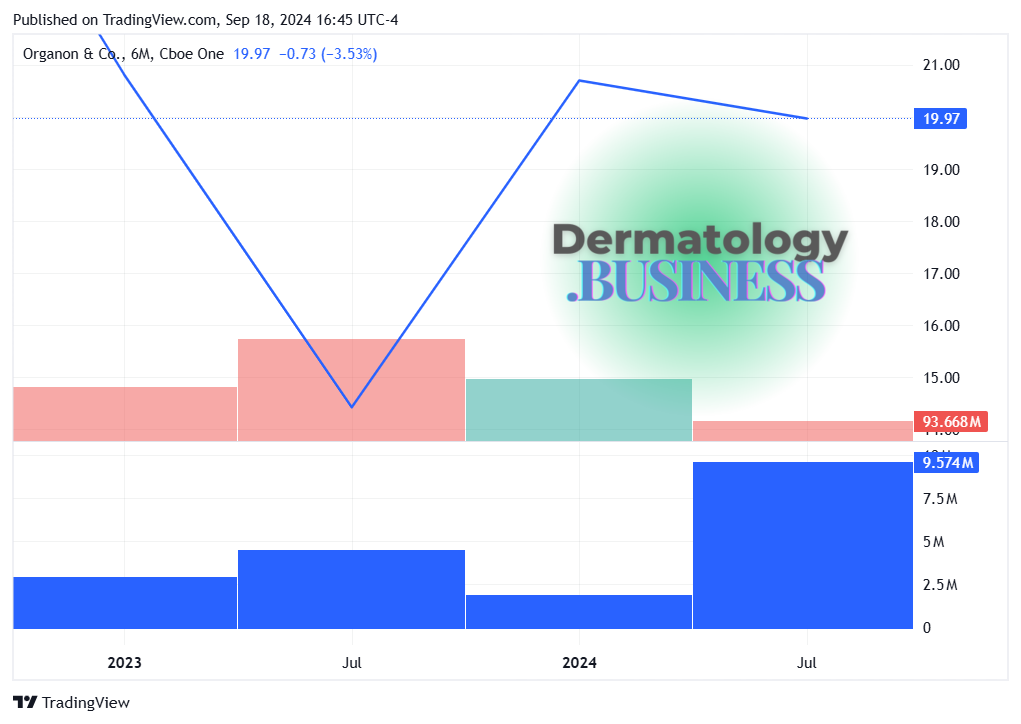
Organon to acquire Dermavant in Cdn$1.6B dermatology play
Organon, the women’s health-focused company spun off from Merck three years ago, is set to acquire Dermavant Sciences for up to Cdn$1.6 billion (approx. US$1.2 billion), the companies announced Wednesday.
Why it matters: The deal expands Organon's dermatology portfolio into the U.S. market and adds VTAMA, a novel non-steroidal cream for plaque psoriasis that could soon be approved for atopic dermatitis.
The big picture: This acquisition aligns with Organon's mission to improve women's health, as both psoriasis and atopic dermatitis disproportionately affect women.
By the numbers
Cdn$235 million upfront payment
Cdn$101 million milestone payment upon regulatory approval for atopic dermatitis
Up to Cdn$1.28 billion in commercial milestone payments
Tiered royalties on net sales
Between the lines: The deal structure is heavily weighted towards success-based milestones, reflecting Organon's commitment to disciplined capital allocation while adding growth assets.
What’s next: The transaction is expected to close in Q4 2024, pending regulatory approval.
It’s expected to be modestly dilutive to Organon's Adjusted EBITDA in 2025, turning accretive in 2026.
Organon’s net leverage is anticipated to rise above 4.0x post-transaction.
Musely’s rapid growth highlights teledermatology market's potential
Teledermatology company Musely reports 100x growth over 5 years, reaching nine-figure revenue.
Inc. 5000 named it the fastest-growing company in its sector.
Why it matters: Musely’s growth trajectory offers insights into the teledermatology market’s dynamics and the potential for digital health platforms to scale rapidly.
By the numbers
Treatments offered expanded from 2 to 17
Claims 50 times lower cash burn rate than competitors
Reports more than 800,000 customer reviews on its website
Between the lines: Musely’s approach combines…
Personalized treatments
Diverse product range (skin, hair, body, hormonal)
Direct-to-consumer model
Challenges ahead
Sustaining growth as the market matures
Potential regulatory scrutiny as the company expands
Managing quality control with rapid scaling
What’s next: Musely plans to launch new treatments and community engagement initiatives.
The bottom line: While Musely’s growth is significant, it remains to be seen how sustainable this model is long-term and how it might impact traditional dermatology practices and pharmaceutical distribution channels.
‘Tattoo regret’ is a real thing and is about to get more real
The big picture: The global tattoo market is booming and is expected to grow from Cdn$2.96 billion ($2.2 billion) to more than Cdn$5.39 billion (US$4 billion) by 2032.
By the numbers
Nearly one-third of American adults have at least one tattoo
More than 20,000 tattoo parlours operate in the USA
The tattoo removal business is projected to reach Cdn$1.08 billion (US$800 million) by 2027
Driving the trend: Tattoos have become mainstream, with people getting inked to memorialize events, express identity, or simply for aesthetics.
Yes, but the saturation of the market has led to increased competition among tattoo artists, making it harder for seasoned professionals to maintain their businesses.
Between the lines: While tattoos are more socially accepted, some still face stigma, particularly older adults with visible ink.
Mapping interest (below): Top Canadian regions searching for the term “tattoo removal” on Google during the past 90 days
The bottom line: As the tattoo industry grows, so does the removal business, indicating that not everyone’s ink is permanent.
Novel tattoo removal cream could challenge laser dominance
A new topical treatment for tattoo removal, developed by Dr. Alec Falkenham of Dalhousie University, might disrupt the tattoo removal market.
Why it matters: The Bisphosphonate Liposomal Tattoo Removal (BLTR) cream offers a potentially safer, less painful, and more affordable alternative to laser removal.
How it works
Targets macrophages that contain tattoo pigment
Uses liposomes as a drug-delivery system
Triggers the natural removal of pigment by the body’s immune system
Key players:
Inventor: Dr. Falkenham (PhD in Pathology)
Commercialization partner: Cipher Pharmaceuticals Inc.
The big picture: With 30% of Americans having at least one tattoo and a growing removal market, BLTR could capture significant market share if successfully commercialized.
What’s next: BLTR is currently in pre-clinical trials. If successful, it’s expected to be available as a prescription medication.
The bottom line: While still in the early stages, BLTR represents a potential paradigm shift in tattoo removal, offering a less invasive option for the millions seeking to erase their ink.
Lack of diversity in clinical trials is still a big problem in derm research
Big picture: Dermatology clinical trials severely lack diversity, mirroring a broader issue across medical research
Why it matters: Diverse clinical trials are crucial for comprehensive data, building trust, and ensuring treatments work for all populations
By the numbers:
74.4% of participants in U.S. dermatology trials were White
Only 30% of U.S. psoriasis studies had more than 20% diverse participants
75% of participants in FDA-approved drug trials in 2020 were White, despite minorities making up 40% of the U.S. population
Driving the news: Dr. Valerie M. Harvey highlighted this issue at the Skin of Color Update 2024 in New York City.
Sure, but: The problem isn’t unique to dermatology.
Prostate cancer: Black men are 2-4 times more likely to die from it, yet are underrepresented in trials
Diabetes: Minorities are 50% more likely to be diagnosed — but only 5% of trial participants were Black
What’s being done:
In Canada, the 10th Annual Skin Spectrum Summit and 4th Annual Indigenous Skin Spectrum Summit will take place Oct. 4 and 5 in Toronto
Skin of Color Society initiatives, including a Diversity Clinical Research Mentoring Program
American Cancer Society collaborating to boost Black men's participation in prostate cancer trials
U.S. FDA developing a diversity action plan for clinical trials
What’s next: Dr. Harvey suggests engaging targeted communities early in trial design to address their concerns and priorities
The bottom line: Increasing diversity in clinical trials is crucial for equitable healthcare outcomes across all populations
Skin Spectrum Summit and Indigenous Skin Spectrum Summit info is at https://skinspectrum.ca
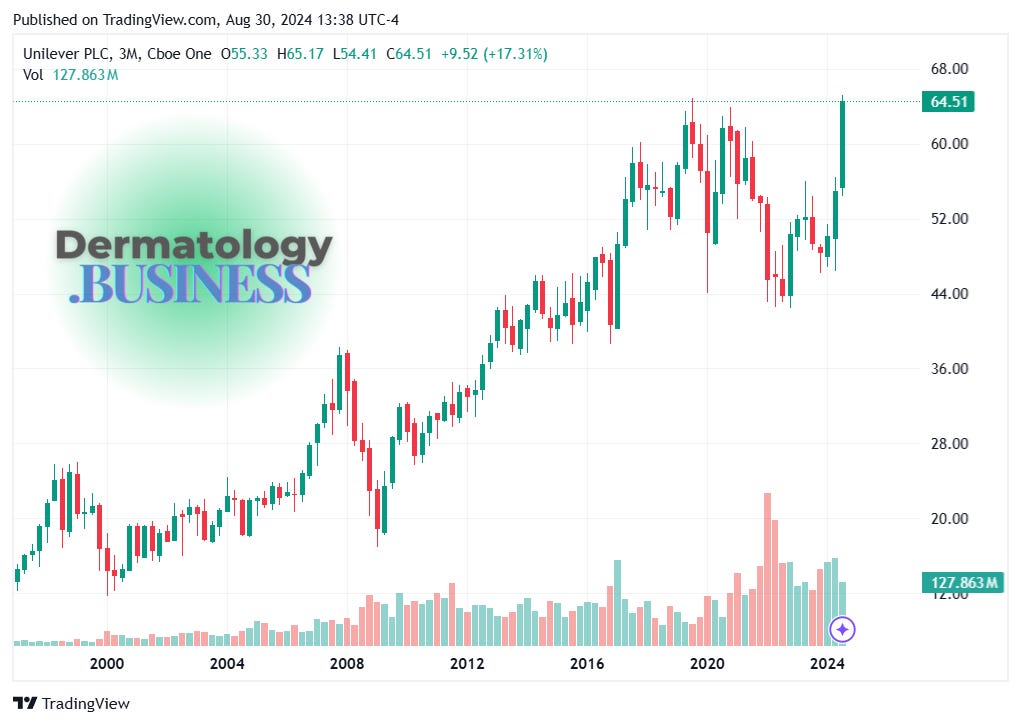
Looking to buy some skin brands? Unilever has a pair to sell
What’s happening: Unilever, the consumer goods giant behind the Dove product line, is looking to sell two of its personal care brands.
Kate Somerville: Upscale skincare brand acquired in 2015
REN: Another skincare brand bought in the same year
Why it matters: This move signals Unilever’s efforts to streamline its portfolio under new leadership.
Behind the scenes
PricewaterhouseCoopers engaged to handle potential Kate Somerville sale
Valuations for the brands not yet disclosed
The big picture: These potential sales come amid broader changes at Unilever.
New CEO Hein Schumacher appointed in July 2023
Plans to demerge or potentially sell its ice cream division (Magnum, Ben & Jerry’s)
What to watch: Whether exiting the niche skin brands might precede divesture of the core Dove franchise.
Tracking Unilever’s 25-year climb
Mobile dermatology startup brings skin cancer Tx to Florida seniors' doorsteps
“Dermatology on the Spot,” a service founded by nurse practitioner Jason Welch, is disrupting the model for skin cancer treatment for Florida patients.
A “you ring, we bring” model?
Welch, who works with dermatologist Dr. Lawrence Blasik, was inspired to start the service after witnessing an elderly patient's transportation struggles.
The startup operates two mobile medical units, a lead-lined van and an RV, to provide radiation and surgical procedures at assisted living centers.
The mobile units have been well-received by assisted living facilities, as they provide prompt service and minimize disruption for skin patients with Alzheimer’s and dementia.
Yes, but: In Canada, where dermatology wait times now routinely exceed a year, the concept of any dermatology HCP following a “30-minutes-or-it’s-free Door Dash” model is unheard of.
Source: Daytona Beach (Fla.) News-Journal
LEO Pharma unveils groundbreaking Chronic Hand Eczema data at ESCD 2024
LEO Pharma presented results from the largest-ever multinational real-world studies on Chronic Hand Eczema (CHE) at the European Society of Contact Dermatitis (ESCD) congress.
Why it matters: CHE prevalence and treatment patterns have been historically understudied. These large-scale studies provide crucial insights into this debilitating condition.
By the numbers
5.6% of study participants self-reported CHE in the last 12 months
21.5% of CHE sufferers attribute the condition to their occupation
12.2% have changed work assignments or reduced working hours due to CHE
90% of moderate to severe CHE patients use medium to ultra-high potency topical corticosteroids
Key findings
CHE is common, affecting about 1 in 20 adults
Significant occupational impact, with 1 in 5 attributing CHE to their job
Persistent symptoms across all treatment groups indicate unmet needs
Over 1 in 4 moderate to severe patients progress to advanced therapies
What’s next: These findings could drive new approaches to CHE management and treatment development.
The bottom line: CHE is more prevalent and impactful than previously understood, highlighting the need for improved treatments and occupational strategies.
Who’s hiring
Eli Lilly Canada is searching for a sales consultant for Eastern Ontario, preferably based in Ottawa (12-month contract)
Ideally, this candidate has experience selling immunology products with dermatologists and allied healthcare professionals. Proven success in previous roles has come from creating exceptional customer experiences, demonstrating a highly collaborative teamwork approach, a winning mindset and strong leadership. The successful candidate will demonstrate omnichannel selling capabilities, which will generate strong connections with customers via virtual and in-person interactions.
Must haves
Good understanding of the Pharmaceutical Sales Consultant role
Immunology (ideally dermatology and/or rheumatology) experience is an asset
Bachelor’s Degree
Completely bilingual: English 1st language and French 2nd language
Hospital and/or key account sales experience
Proficiency with computers, Salesforce and data manipulation/analysis
Recent recognition of leadership, teamwork, customer focus, and performance (i.e., Canadian Pinnacle, President’s Council, District Rep of the Year, or Award of Excellence)
Vivier Pharma needs an account representative for the Toronto East territory
Vivier Pharma is a Canadian Pharmaceutical Skin Care company with over 20 years of experience in the Medical Aesthetic Industry. We are currently looking for an Account Representative to join our Ontario Team. The Account Representative is responsible for uncovering and driving sales for the Vivier Pharma portfolio of products. The focus will be on prospecting, driving new business, and building relationships while maintaining high customer satisfaction. The candidate must live in the Toronto area, as travel will be required to manage accounts in this territory (Yonge St. & East Toronto to Belleville). This is a great opportunity for someone who is passionate about skincare and dermatology and looking for a rewarding career in this growing Medical Aesthetic industry. This is a permanent, full-time remote position.
Responsibilities
Assist in marketing and selling all Vivier product brands in the designated territory in compliance with established monthly, quarterly and yearly strategies
Seek out new business opportunities, create lead generation lists, and close new business opportunities to help meet sales targets
What they’re looking for
University degree, business/science preferred
Minimum two (2) years of previous outside sales experience
Highly motivated and target-driven with a proven track record in sales
LEO Pharma needs a Medical Information Manager
The role is dynamic, pro-active, strategic, and fast-paced, providing a high-quality Affiliate Medical Information service to the Affiliate, Healthcare professionals, patients and other stakeholders. It involves working in close collaboration across the Canadian Affiliate, Global Medical Information (GMI) colleagues and Outsourcing vendor and Healthcare Professionals.
Professional Competencies
Scientific Degree level or equivalent/Pharmacist/Nurse (If higher qualification required for your Country, Affiliate please update)
Significant experience in a Senior Medical Information role in the pharmaceutical industry
Have a strong and high level of scientific knowledge to be able to analyze data from all types of clinical data including Clinical Study Reports and published data in all forms etc
Must have proven strong technical/scientific writing skills and verbal communication including face-to-face with Healthcare Professionals and presenting to a variety of audiences
Proven high-level technical and compliance skills when reviewing LMR materials and authoring SOPs and other high-level process-driven documentation
In-depth knowledge and efficiency in Medical Information systems
Cutera is seeking a Practice Development Manager - Ontario
The Practice Development Manager is responsible for all aspects of customer relationship management and consumable sales in the assigned territory. This includes planning, organizing, and implementing all account management activities related to the sale, onboarding, and utilization of Cutera's key core devices, with high accountability for achieving territory utilization quotas and objectives.
Duties And Responsibilities
Responsible for performing effective territory penetration, coverage, and account identification to drive sales and increase the customer base for consumables
Providing training, presentations, and demonstrations to the customer on the application and use of the company’s products using effective communication and sales techniques
Engage and drive accounts to grow existing business and generate new business opportunities and sales revenue growth
Analyze practice readiness and customize activities to maximize utilization and profitable revenue
Ongoing customer education regarding the indications, contraindications, and safety of Cutera products and how they fulfill the customer’s needs
Bausch Health Companies Inc. is seeking a sales representative for Calgary/S. AB & SK
Contact assigned customer accounts to sell the organization’s products within a designated geographic territory
Focuses on territory sales targets, new business development, and troubleshooting problems or key markets
May have special markets/complex product lines assigned that require significant client relationship skills
Organizes own work routine but guidance is provided by the Regional Sales Manager and the Marketing department
Major Areas of Responsibility
Selling skills/message mastery
Demonstrates an in-depth understanding of their promoted product key messages and consistently delivers to customers in a variety of selling situations
Engage customers in needs-based selling discussions that position our products as a solution to established or identified customer needs
Galderma Canada has several openings, including:
Psst. We’ve got some real estate we think you might be interested in. If you’ve got a message, a campaign, or a service proposition you’d like to communicate to an audience of 1,370 managers, executives and decision-makers in the dermatology space, Dermatology.Business wants to partner with you. We can work with you to create and deploy sponsored content (images, text, video), advertising positions, along with some other stuff you probably haven’t heard of. Get in touch at health@chronicle.org or call 416-916-2476
We hope you enjoyed this issue of Dermatology.Business. We’ll be back to report to you again in two weeks. Meanwhile, stay in touch by letting us know what’s going on in your world: spread the word about your wins, and keep the community posted about job openings, career moves, new pipeline prospects and other items of interest. Please pass the newsletter on to your teams, colleagues, and friends by clicking the link below.
You are receiving this newsletter because you are a client of Chronicle Companies, attended a National Pharmaceutical Congress live event or webinar, or previously requested a subscription to one of our newsletters. If you no longer wish to subscribe to this newsletter, please email the subject line “Unsubscribe Dermatology.Business” to health@chronicle.org.
Dermatology.Business is published by Chronicle Companies, 701 Ellicott Street, Buffalo, NY 14203. Canadian Offices: 1460 The Queensway, Suite 212, Etobicoke, Ont. M8Z 1S4
Mitchell Shannon, Publisher; R. Allan Ryan, Editorial Director; John Evans, Joyce Pitters-Hands, Jeremy Visser, Shannon Abbott, Contributors; Cristela Tello Ruiz, New Business Development; Darren Stallman, Consultant; Amy Elder, Intern
Content is copyright © 2024, Chronicle LifeSci America Corp., except as indicated. Are you interested in contributing to this newsletter or learning about the Chronicle’s services? Please write to us at health@chronicle.org.
Hey, thanks for reading Dermatology.Business. Subscribe for free to receive the next edition.