Aesthetic chain's collapse sends shockwaves through UK cosmetic medicine clinics
Sudden closure of 70 clinics leaves thousands of British patients in the lurch. Plus: Almirall, Galderma H1 results; skincare-obsessed tweens; Cipher goes stateside; Quebec care gaps, and much more
…and we’re back. If this is Wednesday, you must be reading Dermatology.Business. We aim to keep you informed about the latest commercial developments in derm. Spend five minutes with us every other Wednesday, and you'll learn something new. If you like how that deal sounds, why not share this newsletter with your teams and colleagues?
The sudden collapse of Skin, one of the UK’s largest cosmetic surgery providers, has sent ripples through the dermatology and aesthetic treatment sectors. With more than 70 branches across Great Britain, the Birmingham-based company (which styled its brand as “SK:N”) abruptly ceased operations on July 17, 2024, leaving thousands of patients in limbo and hundreds of jobs hanging in the balance.
Skin’s website was replaced with a terse message citing unsuccessful attempts to secure investment for continued operations (see below.) The company, founded in 1990, expanded its footprint across major cities, including Manchester, Liverpool, and Glasgow, with 17 clinics in London alone.
According to the BBC, the fallout from Skin’s demise extends beyond its core business, affecting subsidiaries such as the Harley Medical Group aesthetic clinics and ABC Medical, the distributor of Cynosure, Préime, and Deleo devices. With more than 450 physicians, nurses, and practitioners suddenly out of work, the collapse will raise questions about the management of practices marketing aesthetic treatments.
Patients have been stranded without support, with pre-paid treatments now in jeopardy. The company’s communication channels have gone dark, leaving customers scrambling for refunds and alternative care options.
This development comes as the North American dermatology landscape is trending toward consolidation. Private equity firms and more giant healthcare conglomerates have aggressively acquired independent practices, creating sprawling networks of dermatology clinics across the United States and Canada.
Why this matters: Maintaining patient trust is essential across the entire sector. Once lost due to abrupt clinic closures and unfinished treatments, it will be challenging to regain patient confidence.
The bottom line: While the North American market differs in several respects from the UK’s, the Skin collapse underscores the need for sustainable growth strategies and robust patient protections in an increasingly consolidated dermatology landscape.
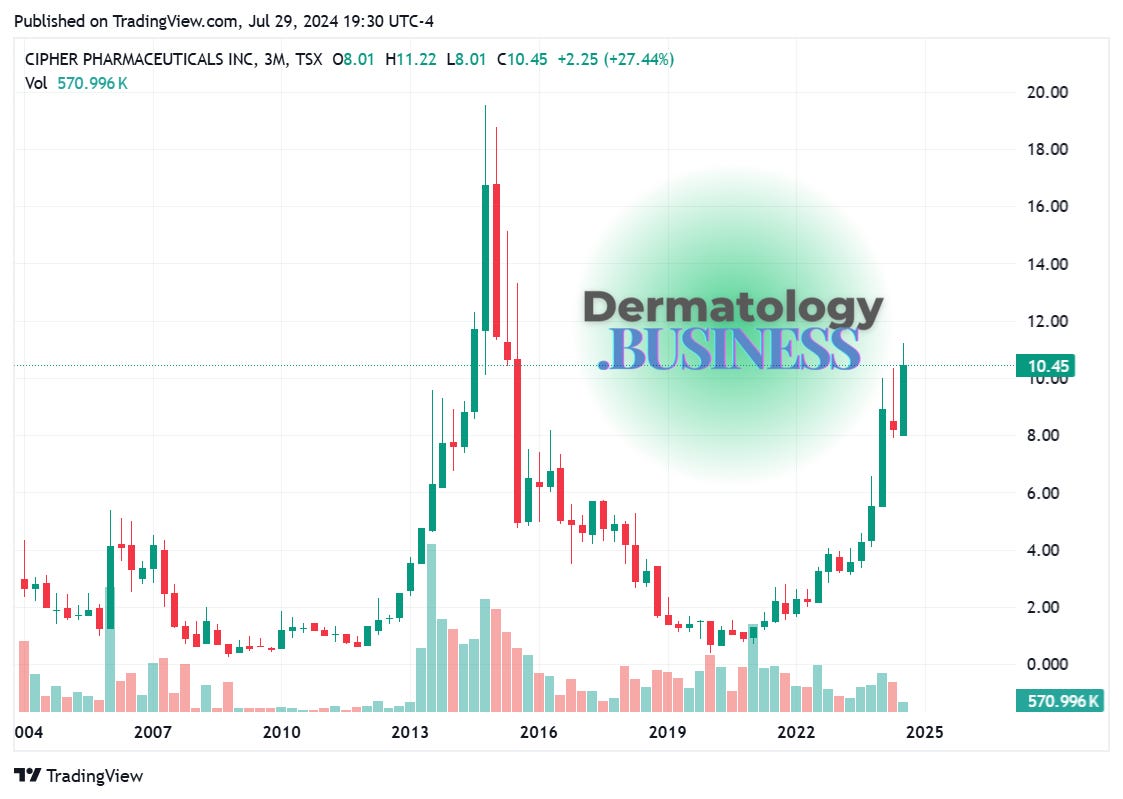
Cipher Pharmaceuticals acquires Natroba for US$89.5M
Cipher Pharmaceuticals has purchased the global rights to spinosad (Natroba), a topical treatment for head lice and scabies, from U.S. drug developer ParaPRO for US$89.5 million.
Why it matters: This acquisition doubles Cipher’s current sales and earnings, re-establishing it as a significant player in the dermatology market.
Details:
Cipher paid US$80 million in cash and US$9.5 million in common shares.
The deal includes ParaPRO's U.S. commercial sales team of 50 employees.
Natroba has FDA exclusivity and patent protection for scabies treatment until 2033.
By the numbers:
In the U.S. anti-parasitic market:
Permethrin 5% holds ~71.4% market share
Natroba (Spinosad) has 22.1% market share
What’s next? Cipher plans to:
Submit Natroba for Health Canada approval later in 2024
Out-license Natroba in countries outside North America
Use ParaPRO’s Indiana office as its U.S. headquarters
The big picture: This acquisition aligns with Cipher’s strategy to build a leading dermatology platform with unique, innovative products. It marks the company's return to the U.S. market following the 2017 divesture of its South Carolina-based subsidiary.
Roller-coaster decade at Cipher
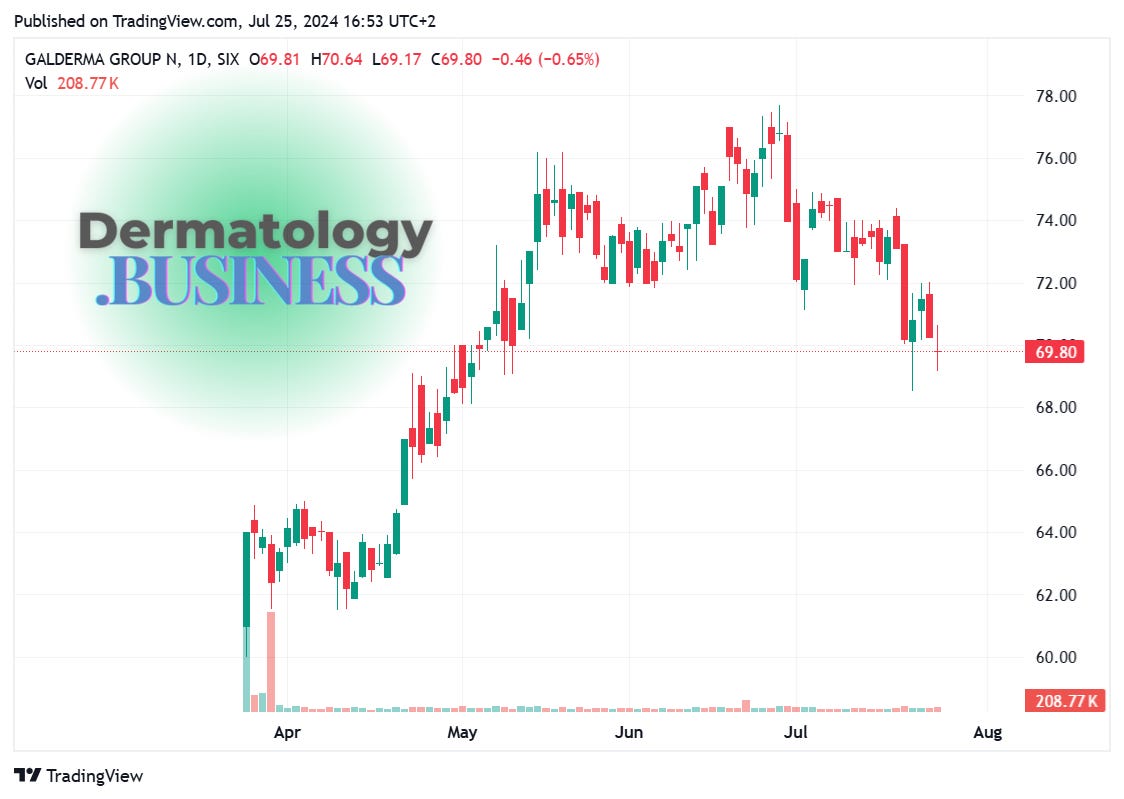
Galderma crushing it, reports strong H1 2024 growth
Why it matters: Galderma’s performance demonstrates the resilience and growth potential in the dermatology market, with implications for competitors and investors in the pharma space.
By the numbers:
Net sales: Cdn$2.95 billion, up 10.8% at constant currency
Injectable Aesthetics: Up 13.4%
Dermatological Skincare: Up 11.8%
Therapeutic Dermatology: Up 2.2%
Core EBITDA: Cdn$689 million, 23.4% margin
Key developments:
First marketing authorization for RelabotulinumtoxinA in Australia
Nemolizumab launch preparations underway
Leverage reduced to 2.6x, CAD 134 million debt repaid early
What’s next: Galderma updates 2024 guidance, expecting net sales growth towards the upper end of 7-10% at constant currency.
Big picture:
Galderma’s integrated dermatology strategy and pipeline progress position it for continued growth in a competitive market.
Separately, the company learned this week that European regulators provided a positive decision for RelabotulinumtoxinA (Relfydess), a new ready-to-use liquid neuromodulator.
Being a public company looks good on Galderma
Spanish drugmaker Almirall posts strong H1 growth
Why it matters: Almirall’s success in European dermatology highlights the potential for targeted therapies in niche markets.
By the numbers:
Total net sales up 6.7% in H1 2024
Revenue reached Cdn$738.5 million, a 3.2% YOY increase
Market cap: Cdn$2.97 billion
Driving the news: Almirall reaffirmed its 2024 EBITDA guidance of Cdn$260 to 282 million, boosting investor confidence.
Key products:
1. Tildrakizumab (Ilumetri, aka Ilumya) for psoriasis:
H1 sales surpassed Cdn$148.5 million, up 25% YOY
2024 peak sales forecast: Cdn$371 million
2. Lebrikizumab (Ebglyss) for atopic dermatitis:
Recently launched in Germany, UK, and Norway
Recommended by NICE for NHS use
What’s next: Later this year, Almirall plans to roll out Ebglyss in Denmark, Spain, and Austria.
The bigger picture: While European dermatology thrives, Almirall faces challenges in the US market due to generic competition.
‘Sephora kids’ trend raises dermatological concerns
A CTV News report highlights growing concerns among dermatologists about the “Sephora kids” trend.
Key takeaways:
Youth are flocking to mall retailer Sephora, showcasing expensive skincare hauls on TikTok
Dermatologists warn adult skincare products may harm pediatric skin
Ingredients like retinol, exfoliating acids, and collagen are unnecessary for young users
Overuse of products could lead to skin barrier damage and potential allergies
Why it matters: This trend represents a significant shift in skincare consumer demographics, potentially opening new markets but raising ethical concerns.
What to watch:
Potential for development of youth-specific skincare lines
Increased demand for dermatologist-approved products for younger demographics
Possible regulatory scrutiny on marketing adult skincare to minors
Bottom line: The “Sephora kids” trend presents opportunities and challenges for the skincare industry, necessitating a balanced product development and marketing approach.
Quebec facing critical derm shortage
Why it matters: Nearly 100,000 Quebecers are waiting for dermatology consultations, highlighting a significant gap in healthcare access that could impact patient outcomes and create opportunities for pharma companies.
By the numbers:
93,000 dermatology consultation requests made in 2023-2024
Average wait time: 198 days
Median wait time: 81 days
Only 200 dermatologists in Quebec's public system
Driving the news: Factors contributing to the shortage include
Insufficient dermatologists for a population of 9 million
Changing work-life balance preferences among doctors
Stagnant consultation fees pushing specialists toward cosmetic procedures
What’s next: The province is exploring teledermatology to reduce in-person visits, with promising results.
The big picture: Addressing this shortage becomes crucial for public health outcomes as the population ages and skin cancer risks increase.
Sun Pharma’s deuruxolitinib gets FDA ok for severe alopecia areata
Why it matters: This US FDA approval for Leqselvi marks the third treatment option for severe alopecia areata in the USA, potentially reshaping the market landscape for autoimmune dermatology. The oral JAK inhibitor baricitinib (Olumiant, Eli Lilly) was FDA approved in June 2022, followed by the JAK3/TEC inhibitor ritlecitinib (Litfulo, Pfizer) in April of this year.
By the numbers:
AA affects ~700,000 people in the U.S., 300,000 with severe cases
>30% of patients achieved ≥80% scalp hair coverage at 24 weeks
Up to 25% of patients in the THRIVE-AA1 and THRIVE-AA2 trials regained ≥90% scalp hair coverage
Key points:
Leqselvi is an oral JAK1/JAK2 inhibitor, offering a new mechanism of action
Approval based on two Phase 3 trials with 1,220 patients
Low discontinuation rate (3.1%) due to adverse reactions
What’s next: Sun Pharma USA will introduce an access program to support eligible patients.
Big picture: This approval strengthens Sun Pharma’s position in the derm market and increases attention on AA, a condition that formularies and payers have been hesitant to cover.
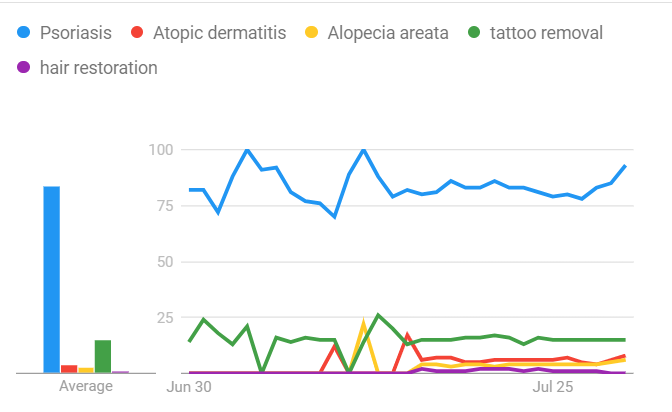
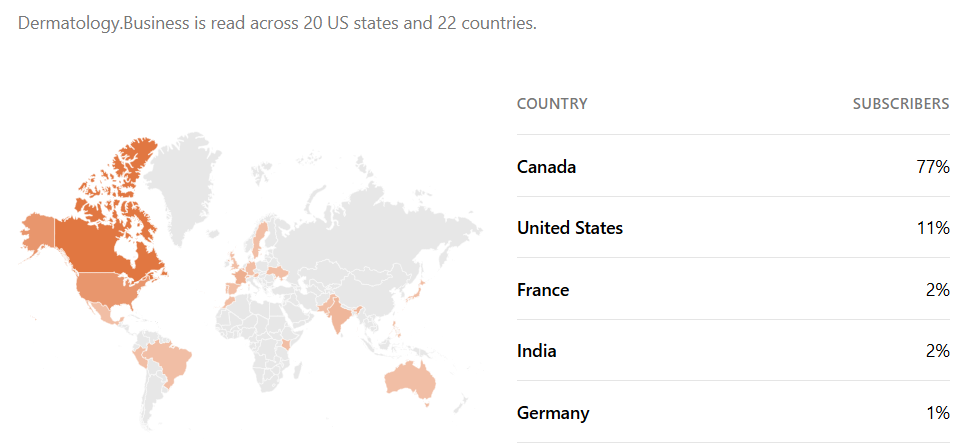
Patient pulse: Dermatology terms Canadians have been searching for on Google during the past 30 days
Stand for ‘skin health for all’: Sponsor the 2024 Colloquium on the Black Patient in Dermatology
For sponsorship information, please get in touch with Shannon Abbott, Joyce Pitters-Hinds, Darren Stallman, or Mitch Shannon. Find the program information at https://skinspectrum.ca
Who’s hiring
LEO Pharma is looking for a Medical Information Manager in Markham, Ont.
Experience
Scientific degree level or equivalent/Pharmacist/Nurse
Significant experience in a Senior Medical Information role in the pharmaceutical industry
Knowledge
Knowledge and experience of working in global and local cross-functional teams
Experience in leading and managing Medical/Scientific Information Booths at Congress
High-level knowledge and understanding of technical reviews within LMR frameworks
Job Responsibilities
Attend the office 2 to 3 days per week
Actively contribute and develop the Global Medical Information strategy and department activities, including participation in GMI projects and tasks as required
Build strong and collaborative relationships with other global LEO Pharma Medical Information colleagues to build synergies and help drive efficiencies.
UCB Pharma needs a Marketing Solutions Lead, Immunology in Oakville, Ont. (Hybrid)
Requirements
A bachelor’s degree in health sciences, business or related fields is required.
Minimum 5+ years of biopharma product management experience with 2+ years of biopharmaceutical sales experience
Experience in specialty / biologic marketing is preferred
Product launch experience is an asset
Bilingual (English/French) is considered an asset; experience in rheumatology and dermatology is preferred
Some travel (15-20%) – Congress, Advisory Board Meetings, and Territory / HCP visits are required
Brunel is currently hiring an Ontario Territory Sales Manager for a medical aesthetics device organization
Responsibilities
Identify and cultivate sales leads
Support sales professionals in achieving territory sales objectives
Maintain regular communication with managers, reporting all developments, including customer interactions, market changes, and competitive activity
Meet or surpass established monthly, quarterly, and annual sales targets
Participate in workshops and trade shows showcasing the client’s product lines
Embody and uphold the ethos of delivering exceptional service every day
Requirements
Bachelor of Science degree is preferred
2+ years of experience in selling capital equipment or B2B sales
Exceptional communication and organizational abilities
Proven track record of customer development
Demonstrated sales achievements
Proficiency in building strong customer relationships
Self-motivated and highly organized
Excellent interpersonal and communication skills
Adequate physical requirements are essential for successfully performing the duties of this role
Why apply through Brunel? Finding the next step in your career can be a full-time job in itself. Brunel manages the process for you: from submitting your resume to coordinating interviews to extending offers and assisting with onboarding. Brunel will get you going while you get on with the job.
Eli Lilly and Company needs a Medical Science Liaison - Immunology-Dermatology (Ontario)
Responsibilities
The MSL will serve as a scientific resource for Canadian experts in Dermatology. It is responsible for responding to requests for in-depth, cutting-edge scientific information from the medical community in the autoimmune field, including the latest scientific literature, research from major scientific conferences, updates on Lilly’s educational activities, and pipeline research activities.
The MSL will also gather feedback and insight from Scientific Experts and Thought Leaders to better inform Lilly’s research and overall strategic direction. By facilitating scientific exchange between the academic community and Lilly, the MSL has the opportunity to shape the future of healthcare with information that addresses important clinical and scientific questions.
Requirements
Advanced degree in health sciences (PhD, MD, Pharm D) with 2-3 years of relevant clinical or therapeutic experience in Immunology - Dermatology
or MSc in health care/professional designation (BScN, PT, RD, etc.) is considered if there are five or more years of relevant clinical experience in immunology-dermatology.
or previous MSL experience and sound knowledge of the pharmaceutical industry
Dermatology/Immunology-Biologics experience
Proven several years of related medical and research and development experience in the pharmaceutical, biotechnology, and medical devices industry